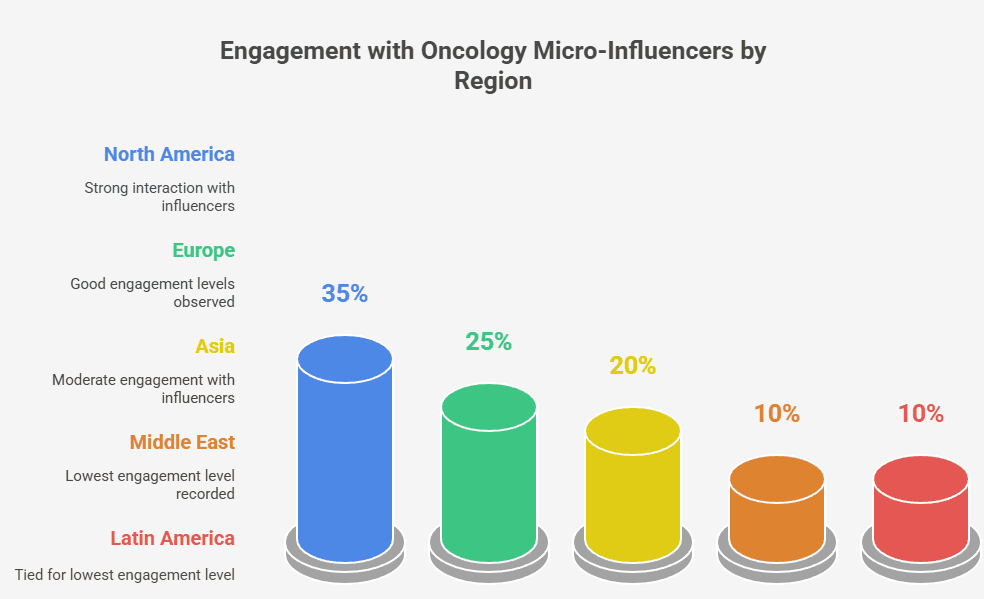
Introduction: Navigating Complexity with Strategic Digital Influence
In today’s oncology landscape, the complexity of cancer care has grown alongside the digital sophistication of healthcare professionals. Pharma marketing, particularly for oncology, can no longer rely on traditional visibility tactics such as banner ads or generic webinars. Oncologists, burdened with clinical, administrative, and emotional responsibilities, expect digital engagement that is both evidence-based and respectful of their expertise and time.
This shift in expectations calls for a reimagining of how pharmaceutical marketers deliver value. Instead of broad promotional messaging, the emphasis must move toward precision—targeted content, ethical influencer collaboration, and measurable educational outcomes.
This article explores modern, global strategies for pharma marketing in oncology—particularly through the ethical use of digital micro-influencers and scientific content. We also examine performance metrics, formats, and goals, supported by data visualization.
The Evolving Role of Pharma in Oncology’s Digital Transformation
Digital transformation in oncology is about more than delivering content—it’s about enabling smarter, faster decision-making for clinicians. Oncologists now engage with a range of tools and platforms: HCP portals, medical apps, live virtual tumor boards, peer-to-peer groups, and short-form educational content on social platforms like LinkedIn and YouTube.
Pharmaceutical marketing, therefore, must evolve from campaign-based promotions to ecosystem-building. This includes curating resources that align with the oncologist’s workflow and complement scientific reasoning, all while adhering to strict regulatory standards.
Harnessing the Influence of Digital Onco-Leaders
A rising trend in oncology marketing is the collaboration with micro-influencers—clinicians who may not be globally known KOLs but who engage deeply with a specific HCP audience. These may include hematologist-oncologists, early-career researchers, or academic faculty known within national networks.
Unlike celebrity KOLs, micro-influencers bring:
- Practical clinical insights from active cases
- Peer relatability
- Niche audience engagement (e.g., sarcoma, pediatric oncology)
- Active presence on digital platforms
This emerging model allows pharma marketers to build credibility through peer-led education, not just brand-led promotion.
Micro-Influencers vs KOLs: When and How to Use Each
While micro-influencers bring agility and peer relatability, traditional Key Opinion Leaders (KOLs) still play an irreplaceable role in shaping broad clinical consensus, especially in product launches and international guidelines.
When to use KOLs:
- Launch of first-in-class therapies
- Regulatory milestone events (FDA/EMA/DCGI approvals)
- Endorsement of international consensus or multicenter trials
When to engage micro-influencers:
- Post-launch education and follow-up
- Localization of clinical pathways
- Addressing FAQs, myths, or post-market concerns
By combining the credibility of KOLs with the accessibility of micro-influencers, pharma marketers create a hybrid strategy that touches both ends of the HCP influence spectrum.
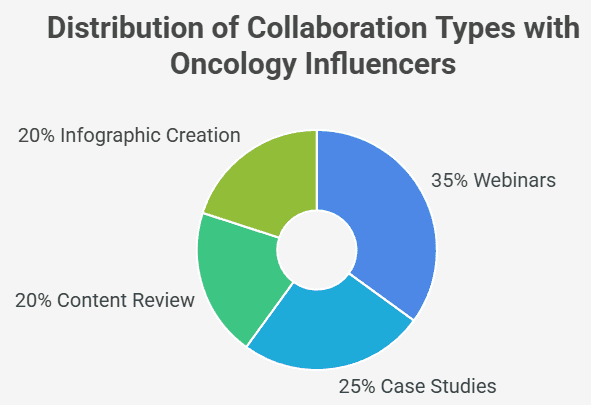
Interpretation:
The chart above illustrates that webinars (35%) and case studies (25%) form the bulk of influencer collaborations, reflecting a preference for content formats that are both clinical and interactive. Infographic creation and content reviews support quick updates and evidence translation.
Key Elements of Ethical Collaboration
To collaborate successfully with oncologist influencers, pharma brands must follow principles rooted in transparency and mutual value.
1. Co-creation over control:
Let the influencer develop content in their own voice, grounded in evidence and clinical relevance. Avoid over-branding.
2. Value-driven relationships:
Provide incentives such as access to real-time research, conference participation, authorship opportunities, or featured roles in CME portals—not just honoraria.
3. Regulatory transparency:
Clearly disclose sponsored content. Avoid superiority claims, and strictly avoid off-label promotion.
4. Platform fit:
Use each influencer’s strengths: e.g., LinkedIn for case slides, X (formerly Twitter) for trial commentaries, YouTube for explainer videos.
The Role of Data Privacy and Regional Compliance in Oncology Marketing
In the highly regulated field of oncology, trust is not only clinical—it’s also legal and ethical. As digital strategies become more personalized, pharma marketers must account for region-specific data privacy regulations. From Europe’s GDPR to India’s DPDP (Digital Personal Data Protection) Act, maintaining compliance in campaigns involving HCP data, patient testimonials, or influencer-generated content is paramount.
Best practices include:
- Using opt-in mechanisms for email and webinar registrations.
- Hosting digital tools on secure, regionally compliant platforms.
- Disclosing the intended use of interaction data, especially with gated content.
- Including local disclaimers and regulatory information in influencer posts.
Campaign success isn’t just about engagement—it’s about ensuring that the HCP relationship is never compromised by perceived data misuse.
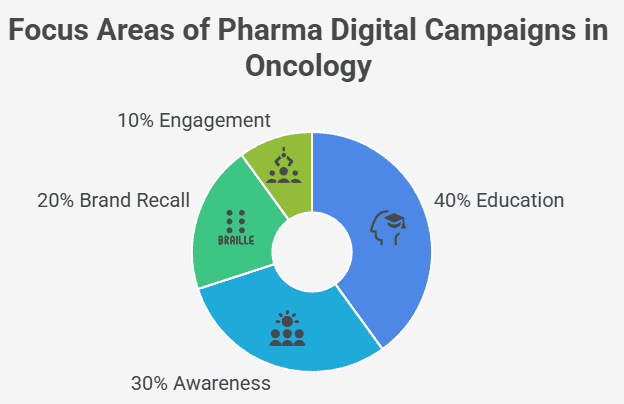
Interpretation:
As the chart shows, education (40%) remains the primary objective of pharma marketing via digital influencers, followed by engagement (30%). This emphasizes the trend toward building clinically supportive relationships rather than pushing product visibility.
Digital Content That Resonates with Oncologists
Pharma content strategies should prioritize micro-learning, clinical usability, and interactivity. The following formats are most effective:
Clinical explainers: MOA (mechanism of action) animations, adverse event breakdowns
- Interactive algorithms: Personalized by biomarker or patient subgroup
- WhatsApp-shareable resources: Consent checklists, multilingual patient tools
- ESMO/NCCN guideline summaries: Short, mobile-friendly recaps with local relevance
A single well-crafted, 2-minute video on managing neutropenia may outperform an entire static campaign in terms of HCP engagement and retention.
Case Spotlight: Oncology Biosimilar Launch Using Peer Education
A pharmaceutical company aiming to improve biosimilar acceptance among Indian oncologists collaborated with six regional micro-influencers across teaching hospitals. Rather than branded materials, they co-developed:
- Myth-busting infographics
- Peer-reviewed explainers on efficacy equivalence
- Regional-language videos addressing safety concerns
Within 90 days, the campaign saw:
- 4X higher engagement compared to traditional emails
- 21% increase in clinical resource downloads
- Over 120 new HCP inquiries
This approach proved that education-first storytelling outperforms direct product marketing, especially in sensitive domains like biosimilars.
Interpretation:
The bar chart indicates how content views and downloads dominate performance metrics, while repeat visits and inquiries are valuable indicators of campaign trust and educational value. These metrics suggest that oncologists return to content they find genuinely useful, reinforcing the importance of relevance over reach.
Measuring What Matters: From Clicks to Clinical Impact
In pharma marketing for oncology, vanity metrics like impressions have limited value. Instead, focus on:
- Time spent on content: Are oncologists actually reading or watching?
- Download and tool reuse frequency
- Follow-up actions: e.g., rep inquiries or peer sharing
- Survey-based sentiment analysis
- Portal revisit patterns: A strong indicator of value
Every piece of content should not only inform—it should enhance a clinical decision.
Conclusion: From Brand Awareness to Practice Enablement
The future of pharma marketing in oncology is not about the loudest voice—it’s about the most clinically supportive one. Campaigns that enable better diagnosis, patient communication, or therapy sequencing will always outlast those that chase visibility alone.
To succeed in this landscape, pharma marketers must:
- Co-create with credible digital voices
- Align campaign goals with clinical relevance
- Use platforms that match oncologist behavior
- Measure outcomes that reflect impact, not just attention
From India to Europe, from webinars to WhatsApp, the new era of oncology marketing is collaborative, educational, and ethical.
The Oncodoc team is a group of passionate healthcare and marketing professionals dedicated to delivering accurate, engaging, and impactful content. With expertise across medical research, digital strategy, and clinical communication, the team focuses on empowering healthcare professionals and patients alike. Through evidence-based insights and innovative storytelling, Hidoc aims to bridge the gap between medicine and digital engagement, promoting wellness and informed decision-making.



